Lab 5 Phagocytosis and Microbial Killing by Macrophages
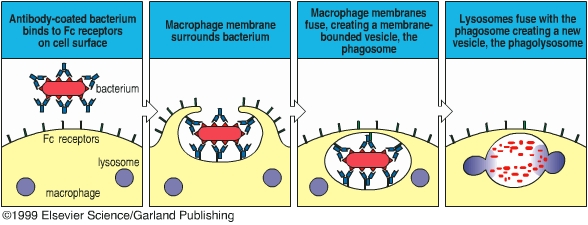
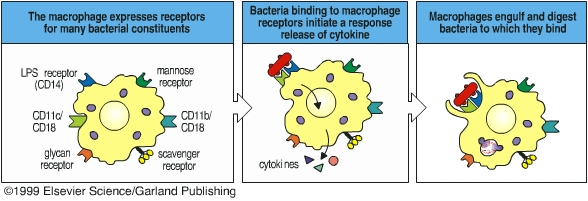
Phagocytes ingest and destroy foreign particles (such as bacteria) and dead host cells. Phagocytes form a vitally important front-line defense against infection and are a major part of the innate immunity. The Russian scientist Elie Metchnikoff first described the phenomenon of phagocytosis in the late 1800’s, and he has been considered as the father of phagocytosis. Phagocytic cells include neutrophils and macrophages. The major phagocytic cell of the blood stream is the neutrophil. The major phagocytic cell of the tissues is the macrophage. Macrophages arise from monocytes in the bloodstream. Phagocytes migrate out of the blood vessels to the infection sites where neutrophils and macrophages phagocytose pathogens and clear out the infection. Phagocytes bear several different receptors that recognize microbial components and induce phagocytosis. CD14 is a receptor for lipopolysaccharide, mannose receptor, scavenger receptor, and glucan receptor bind bacterial carbohydrates. Antibodies and complement components can facilitate phagocytosis by opsonizing pathogens. Antibody- or complement-opsonized particles are phagocytosed by Fc receptor- or complement receptor-mediated pathway.
In this exercise you will determine the ability of macrophages to kill bacteria. We will incubate bacilli with mouse macrophages for 25 min at 37oC to allow macrophages to phagocytose the bacteria. After washing off the unbound bacteria, the macrophages are incubated at 37oC for 60 min. During this time, macrophages kill the ingested bacteria. We will determine the number of viable bacteria before and after the second incubation and observe macrophage-ingested bacteria under a microscopy. A decrease in the number of macrophage-associated, viable bacteria reflects macrophage-mediated bacteria killing.
Procedure
Feb. 4
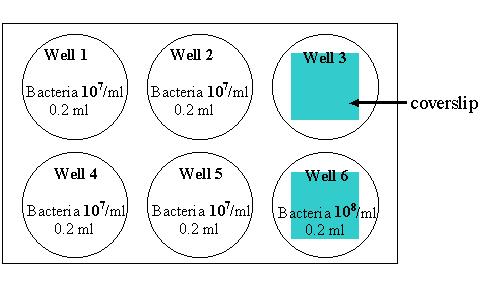
1. Macrophages have been cultured in a 6 well plates. Add the following things to the 6-well plate containing macrophages and 2 ml HBSS:
2. Incubate at 37°C for 25 min to allow macrophages to internalize bacteria. 3. Wash the cells with cold HBSS for 3 times to remove bacteria that are not associated with macrophages. To do this, remove the solution from each well using a transfer pipette and dispose the solution containing bacteria to an appropriate container. Add ~2 ml HBSS to each well, gently swirl the plate a few times and then remove the HBSS. Repeat this twice. Please separate the pipette for adding HBSS from the one for removing HBSS.
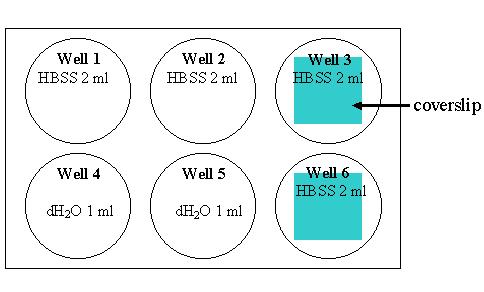
4. Add the following things to each well as indicated in the table:
5. Water lyses the macrophages and release the bacteria in the well 4 and 5. Using p1000 pipetteman pipette up and down a few times to make sure all cells are lysed, then transfer the cell lysate to an eppendorf tube with an appropriate label (an eppendorf per well). Using well 4 and 5, we will determine the number of macrophage-associated bacteria before the killing. 6. Remove coverslips from well 3 and 6 onto a microscope slide in a petri dish. Mark the left corner of the microscope slide and put the coverslips from well 3 on the left side of the slide close to the mark. Please add more HBSS onto the coverslips, and do not let the cells dry out.
7. Incubate the cells in well 1 and 2 at 37oC for 1 h. During this time, macrophages start to kill internalized bacteria.
8. During the 1 h incubation, each group of 4 students will be divided into 2 groups.
GROUP 1
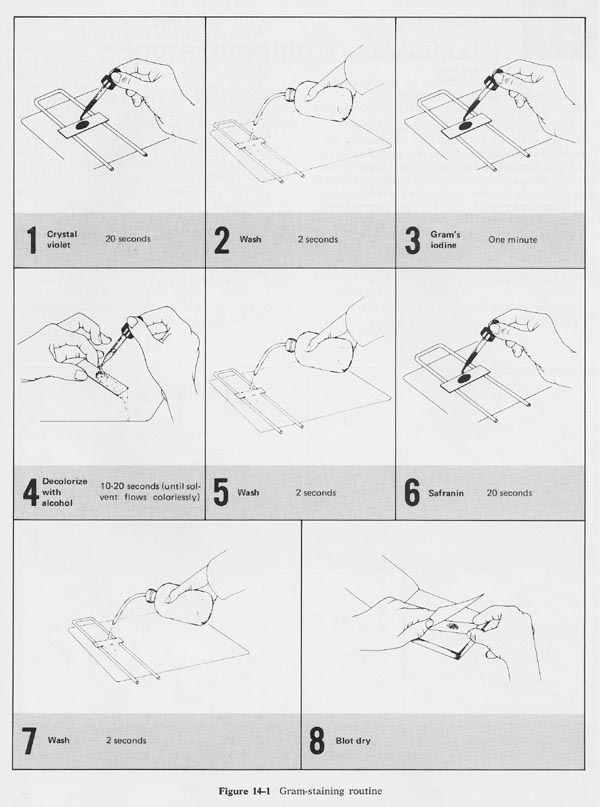
Carry out gram stain of the cells on the coverslips and look for phagocytosed bacteria within cells under a microscope.GROUP 2
Make ten-fold serial dilutions of the cell lysates from well 4 and 5. To do this, set up 4 eppendorf tubes with proper labels (such as 4-1, 4-2, 4-3, and 4-4) for each sample. Add 0.9 ml dH2O to each tube. After vortex the cell lysate, transfer 0.1 ml to the first tube, mix by vortex, and then transfer 0.1 ml from the first tube to the second tube. Continue making ten-fold dilution through the forth tube.
Label 4 agar plates for each sample with the well number and dilution factor, such as 4-1:10, 4-1:100, 4-1:1000, and 4-1:10000. After vortex, transfer 0.1 ml from the first dilution tube to an agar plate labeled “4-1:10”. Spread the inoculum on the plate using a bent glass rod that has been dipped in ethanol and passed through the Bunsen burner flame. Be sure to allow the rod to cool before placing it in the inoculum. Keep spreading the inoculum until it has been completely absorbed by the agar. Continue spreading 0.1 ml from each dilution onto the appropriate plate, flaming the glass rod between each plate.9. By the end of 1 h incubation, remove the HBSS from well and add 1 m dH2O to well 1 and 2. Using p1000 pipetteman pipette up and down a few times to lyse the cells, and then transfer the cell lysates to eppendorf tubes. Using well 1 and 2, we will determine the number of viable bacteria after the second 37oC-incubation.
GROUP 1
Make ten-fold serial dilutions of cell lysate from well 1 and 2 and inoculate agar plates as described above.GROUP 2
Observe the cells on coverslips under a microscope.10. Invert the plates and incubate them at 37oC for 24 to 48 h.
Feb. 6
11. Count the number of colonies on countable plates (30 to 100 colonies/plate) and calculate the percentage of viable bacteria.
# colonies Dilution Well 1 Well 4 % viable bacteria 1:10 1:100 1:1000 1:10000
# colonies Dilution Well 2 Well 5 % viable bacteria 1:10 1:100 1:1000 1:10000
Study Questions
1. What is the function of phagocytes in innate immunity?
2. What are two differences between macrophages and neutrophils?
3. List four different receptors that are expressed by phagocytes and induce phagocytosis.
4. How does the acquired immunity help the innate immunity to clear out infections?